Summary
Pharmacokinetic and pharmacodynamic properties of a new controlled-release (CR) formulation of metoprolol1 have been compared with those of atenolol2. Metoprolol CR (100 mg and 200 mg), atenolol (50 mg and 100 mg) and placebo were each given once daily for four days in a double-blind, cross-over study to ten healthy men.
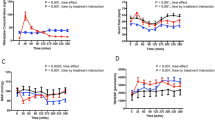
The plasma concentration-time profiles were more even with metoprolol CR than with atenolol over the 24-h dose interval, shown by the lower fluctuation ratio and the longer time period during which the plasma concentration exceeded 50% of the maximum concentration.
All four active treatment regimens reduced exercise heart rate over the 24-h period compared with placebo. However, the reduction in both exercise heart rate and systolic blood pressure (SBP) was more even with metoprolol CR than with atenolol. The remaining β1-blockade after 24 h, expressed as the percentage reduction in exercise heart rate in relation to placebo, was significantly greater after the administration of metoprolol CR, 200 mg, than after either dose of atenolol. At this time the β1-blockade with metoprolol CR, 100 mg, was similar to that with atenolol, 100 mg.
At peak plasma concentrations, 4 h after the dose, the subjects experienced less fatigue during exercise with metoprolol CR than with atenolol.
Similar content being viewed by others
References
Ragnarsson G, Sandberg A, Jonsson UE, Sjögren J (1987) Development of a new controlled release metoprolol product. Drug Dev Ind Pharm 13: 1495–1509
Sandberg A, Blomqvist I, Jonsson UE, Lundborg P (1988) Pharmacokinetic and pharmacodynamic properties of a new controlled-release formulation of metoprolol: A comparison with conventional tablets. Eur J Clin Pharmacol 33 [Suppl]: S9–S13
Ervik M, Kylberg-Hanssen K, Johansson L (1986) Determination of metoprolol in plasma and urine using high-resolution gas chromatography and electron-capture detection. J Chromatogr 381: 168–174
Ervik M, Kylberg-Hanssen K, Lagerström P-O (1980) Electron-capture-gas chromatographic determination of atenolol in plasma and urine, using a simplified procedure with improved selectivity. J Chromatogr 182: 341–347
Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 3: 92–98
Skelly JP and Barr WH (1985) Biopharmaceutic considerations in designing and evaluating novel drug delivery systems. Clin Res Pract Drug Reg Affairs 3: 501–539
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blomqvist, I., Westergren, G., Sandberg, A. et al. Pharmacokinetics and pharmacodynamics of controlled-release metoprolol: A comparison with atenolol. Eur J Clin Pharmacol 33 (Suppl 1), S19–S24 (1988). https://doi.org/10.1007/BF00578408
Issue Date:
DOI: https://doi.org/10.1007/BF00578408